YOUR BROWSER IS OUT-OF-DATE.
We have detected that you are using an outdated browser. Our service may not work properly for you. We recommend upgrading or switching to another browser.
Date: 10.08.2025
New Publication – Synthesis and Reactivity of Alkoxyallene Anions
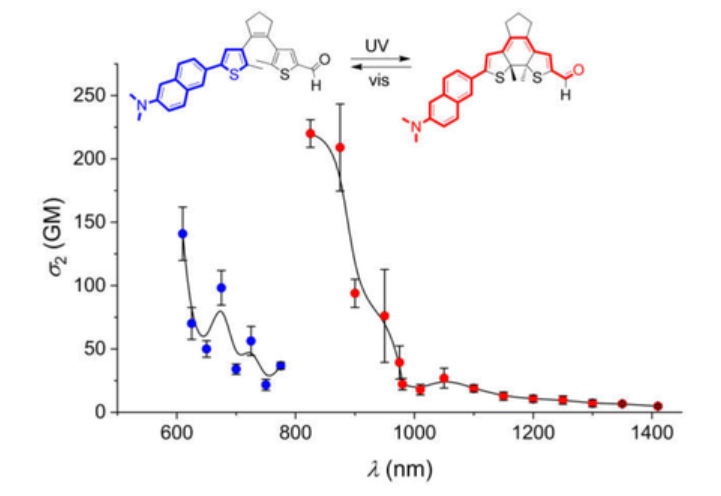
In this work the structural and electronic integration of push–pull systems with photoactivatable dithienylethenes (DTEs) is demonstrated. The light-induced 6π-electrocyclization of two asymmetrically substituted DTEs is used to conjugate an electron-donor (N,N-dimethylaminonaphthyl) and an acceptor part (aldehyde or trifluoromethyl) in the ring-closed form of the photoswitch. This has consequences for the two-photon absorption (2PA) properties, showing substantial shifts toward the near-infrared spectral region for the conjugated push–pull isomer (e.g., 140 GM at 600 nm for the ring-open isomer vs. 220 GM at 840 nm for the ring-closed isomer for the compound with an aldehyde acceptor in THF solvent; measured by Z-scan technique). The possibility to address the dithienylethene core in an all-optical (irradiation at 365 nm for ring-closing and at >550 nm for ring-opening) and reversible manner with good fatigue resistance (≈85%–90% signal recovery after seven switching cycles) provides a straightforward means to control 2PA properties. The experimental observations are rationalized with theoretical calculations (RI-CC2/cc-pVDZ level of theory), providing compelling evidence for the charge-transfer nature of the 2PA-active S0 → S2 transition.